
demonstrated that cavernous tissues contained smooth
muscle and collagen tissues
( Fig. 4e–4 h). Giemsa stain,
which identifies infiltrating leukocytes
[19] ,demonstrated a
qualitative increase in positive cells in cavernous tissues
cultured with allogenic PBMCs compared with tissues
cultured in media alone, autologous PBMCs, and tissues
treated with CsA. Similarly, qualitatively more apoptotic
cells, assessed by TUNEL stain, were observed in cavernous
tissues cultured with allogenic PBMCs compared with the
nonrejecting groups and was reduced in rejecting tissues
treated with CsA.
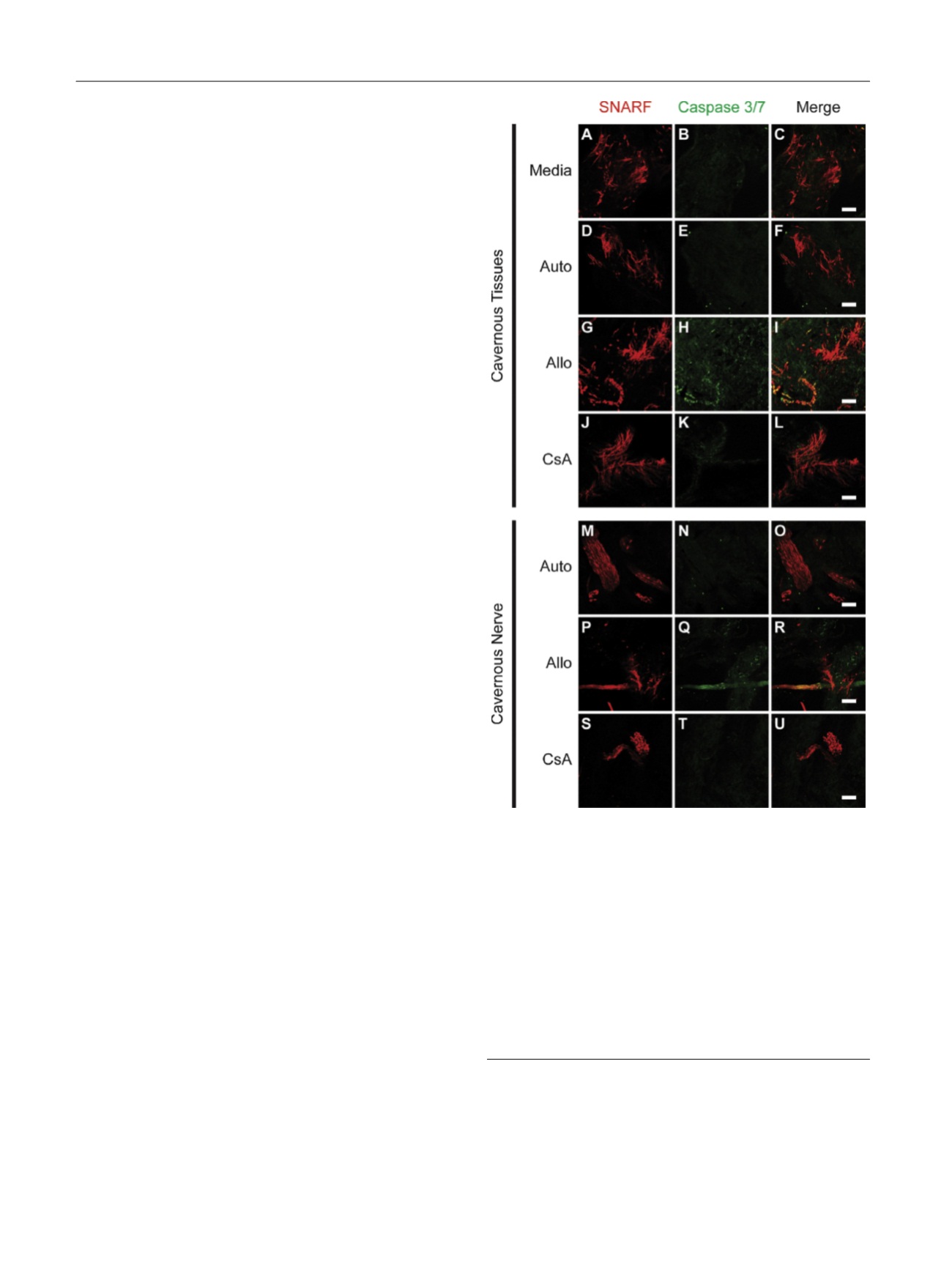
To better understand which tissues were undergoing
apoptosis, we utilized live laser confocal fluorescent
imaging, which enables real-time investigation of tissue
viability and apoptosis using physiologically specific dyes
[14,20]. The tissue is imaged whole without fixation, which
provides the ability to scan through large volumes of tissues
to find rare structures such as cavernous nerves. Using this
technique, we observed both smooth muscle bundles and
cavernous nerve tissues labeled with SNARF-1 undergoing
apoptosis after 48 h of culture with allogenic PBMCs, as
demonstrated by increased activation of caspase 3 and
7
( Fig. 5 ). Apoptosis was prevented when these tissues were
treated with CsA and was not observed in tissues cultured
with autologous PBMCs or media alone.
3.4.
Immunosuppression-specific impairment of ex vivo
corporal reactivity
CsA immunosuppression prevented PBMC activation, tissue
infiltration, and apoptosis in cavernous tissues cultured
with allogenic PBMCs. To determine whether CsA immu-
nosuppression resulted in improved smooth muscle func-
tion, EFS myography was performed on cavernous tissues
cultured with allogenic PBMCs with and without 1
m
M CsA
treatment
( Fig. 6 ). No difference in EFS-mediated contrac-
tion was observed between CsA-treated and untreated
tissues
( Fig. 6 a) (
p
= 0.146). Following maximal precontrac-
tion with PE, CsA-treated cavernous tissues demonstrated
impaired EFS-mediated smooth muscle relaxation com-
pared with untreated, rejecting cavernous tissues
( Fig. 6b)
(
p
= 0.025). Given these unexpected findings, we investi-
gated whether the immune suppression treatment alone
without the influence of PBMCs affected smooth muscle
physiology. Cavernous tissues were cultured without
PBMCs in media alone, with 1
m
M CsA, or with 20 nM
FK506 for 24 h
( Fig. 6c and 6d). No differences in EFS-
mediated contraction were observed between groups
( Fig. 6 c) (
p
= 0.384); however, significant differences were
seen in EFS-mediated relaxation following maximal pre-
contraction with PE
( Fig. 6d) (
p
= 0.005), with CsA-treated
tissues having the least change in contractile force as
measured by percentage of maximal contraction.
4.
Discussion
Penile transplant is a treatment option for severe forms of
disfigurement, which can result from wartime injuries,
traumatic accidents, cancer treatment, and congenital
defects. How the various tissues composing the penis are
affected by allograft rejection and immunotherapy is
unknown. Approaches to model penile transplantation
in animals preclude the ability to accurately assess erectile
physiology
[21–26] .Thus, we used an ex vivo MLR
using PBMCs and cavernous tissue to model human
[(Fig._5)TD$FIG]
Fig. 5 – Live laser confocal microscopy of penile tissues cultured in
media and mixed lymphocyte reaction (MLR) with autologous
peripheral blood mononuclear cells (PBMC; Auto), allogenic PBMCs
(Allo), and allogenic PBMCs treated with 1
m
M cyclosporine A (CsA) for
48 h. SNARF-1 was used to identify living cavernous tissues, and
activated caspase-3/7 was used to identify cells undergoing apoptosis.
Cavernous tissues (a–l) demonstrated (g–i) increased apoptosis in the
setting of rejection (Allo) compared with (a–c) media and (d–f) Auto.
(j–l) Allo-associated apoptosis was prevented by CsA treatment.
Cavernous nerves were found in Auto, Allo, and CsA penile tissues.
Similar to cavernous tissues, (p–r) nerve tissue demonstrated increased
apoptosis in the setting of rejection (Allo) compared to (m–o) Auto,
which was prevented by (s–u) CsA treatment. Bar represents 100 uM.
Allo = allograft mixed lymphocyte reaction; Auto = autologous mixed
lymphocyte reaction; CsA = 1-
m
M cyclosporine A–treated allograft mixed
lymphocyte reaction.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 8 4 – 5 9 3
590
















