
recruitment, and T-cell activation, including increased T
helper cell (Th) 1 and Th2 markers in rejecting tissues,
which was largely prevented by CsA treatment. These data
support the hypothesis that the MLR recapitulates the
complex interaction of innate and adaptive immune
systems with allogenic tissue. In addition, flow cytometry
of PBMCs exposed to allogenic cavernous tissues treated
with CsA had significantly decreased proliferation indices
compared with nontreated PBMCs exposed to allogenic
cavernous tissues (1.16 0.09 vs 6.48 0.37,
p
<
0.001)
(data not shown).
3.3.
Histological characterization of rejecting tissues
Given the decrease in nerve-mediated smooth muscle
contraction and relaxation measured by EFS myography,
we subsequently assessed penile tissue architecture via
histomorphology
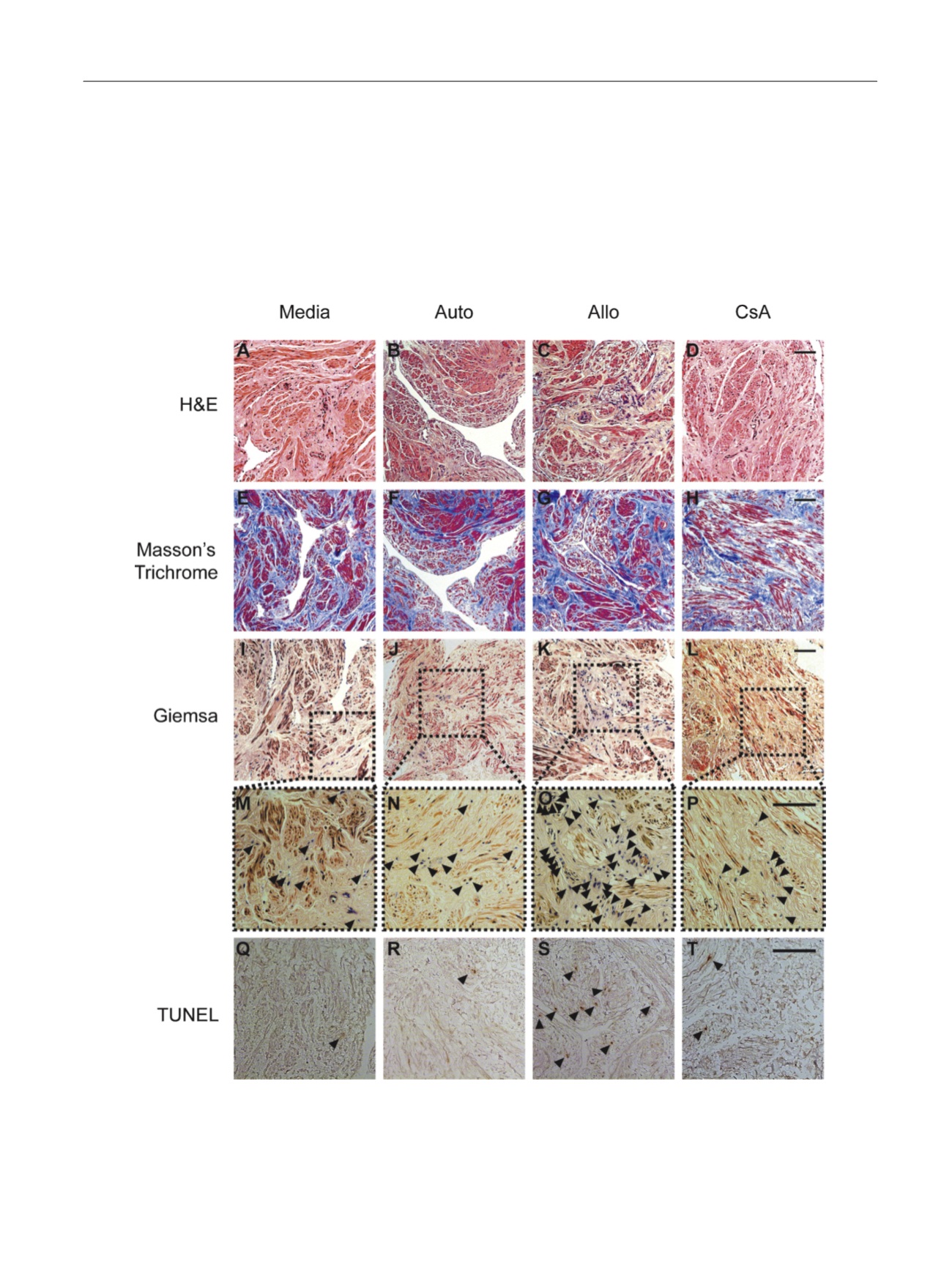
( Fig. 4a–4d). Histochemical evaluation
[(Fig._4)TD$FIG]
Fig. 4 – Representative histology of human cavernous tissue cultured for 48 h in control (media) and mixed lymphocyte reaction (MLR) conditions.
(a–d) Representative hematoxylin and eosin and (e–h) Masson’s trichrome staining of tissue groups demonstrating smooth muscle and connective tissue
content. (i–l) Giemsa stain of inflammatory cells with outlined high-power fields (m–p) demonstrating a qualitative increase in the presence of
inflammatory cells in the allograft MLR (Allo) compared with media, autologous MLR, and 1-
m
M cyclosporine A–treated Allo. (q–t) Terminal
deoxynucleotidyl transferase dUTP nick-end labeling stain demonstrating a qualitative increase in apoptotic nuclei in Allo tissues. Bar represents 100
m
M.
Allo = allograft mixed lymphocyte reaction; Auto = autologous mixed lymphocyte reaction; CsA = 1-
m
M cyclosporine A–treated allograft mixed lymphocyte
reaction; H&E = hematoxylin and eosin; TUNEL = terminal deoxynucleotidyl transferase dUTP nick-end labeling.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 8 4 – 5 9 3
589
















