
84-gene profiler array (Qiagen, PAHS-166ZC) was performed
using a StepOnePlus thermocycler (Thermo Fisher Scientif-
ic). Results were analyzed using the
DD
C
T
method. Data were
imported into Thermo Fisher Scientific’s PCR Array Data
Analysis Web Portal, which provided bioinformatics and
pathway analysis
[15].
2.5.
Myography experiments
Physiological function of human cavernous tissue was
assessed in a muscle strip myograph (Danish Myograph
Technology, 820 M; Aarhus, Denmark), as described
previously
[16,17]. Corporal tissues measuring approxi-
mately 1 2 cm were mounted and bathed in Krebs
solution, maintained at 37
8
C, and bubbled with a mixture
of 95% O
2
and 5% CO
2
. Contractile response to electrical
field stimulation (EFS) was measured with frequencies
of 4.0, 8.0, 16.0, and 32 Hz for 10 s at 40 V, 2.0 ms (Grass
S88 Stimulator; Grass Medical Instruments, Quincy,
MA, USA). Following precontraction by phenylephrine
(PE; 3.0 10
5
M; Sigma-Aldrich), relaxations of penile
sections to EFS were recorded with frequencies of 2.0, 4.0,
8.0, 16.0, and 32.0 Hz for 10 s at 40 V, 2.0 ms, to evaluate
parasympathetic-mediated relaxation
[18] .The force
generation was recorded with AD Instruments PowerLab
8/30 acquisition hardware and LabChart 7 software (ADI
Instruments, Colorado Springs, CO, USA). Cavernous
tissues were weighed after myograph experiments.
2.6.
Statistical analysis
Data are expressed as mean plus or minus standard error of
the mean. The differences between the multiple groups
were compared using a two-way analysis of variance
(GraphPad Prism 6; San Diego, CA, USA). The Student
t
test
was used to compare the difference between two groups.
A
p
value
<
0.05 was considered statistically significant.
3.
Results
3.1.
Rejection impairs cavernous tissue function
An ex vivoMLR combining human cavernous tissue obtained
during penile prosthesis surgery with PBMCs was used to
better understand the process of rejection on cavernous
tissue function
( Fig. 1 ). Penile transplantation was modeled
by combining cavernous tissues with allogenic PBMCs
(ie, cavernous tissues from one person [donor] cultured
with PBMCs from another [recipient]). Cavernous tissues
cultured in media alone or with autologous PBMCs served as
controls. To determine whether our MLR modeled these
conditions, we assessed PBMC activation, which is measured
by proliferation
( Fig. 1), and compared the proliferation
indices (data not shown). PBMCs cultured with allogenic
cavernous tissue had a significantly higher proliferation
index (6.48 0.37) compared with PBMCs cultured in media
alone (3.85 0.34,
p
<
0.001) or PBMCs cultured with
autologous cavernous tissues (3.16 0.11,
p
<
0.001). No
significant difference in proliferation indices was observed
between PBMCs cultured in media and PBMCs cultured with
autologous cavernous tissues, suggesting that PBMCs are not
activated in this model without exposure to allogenic tissue.
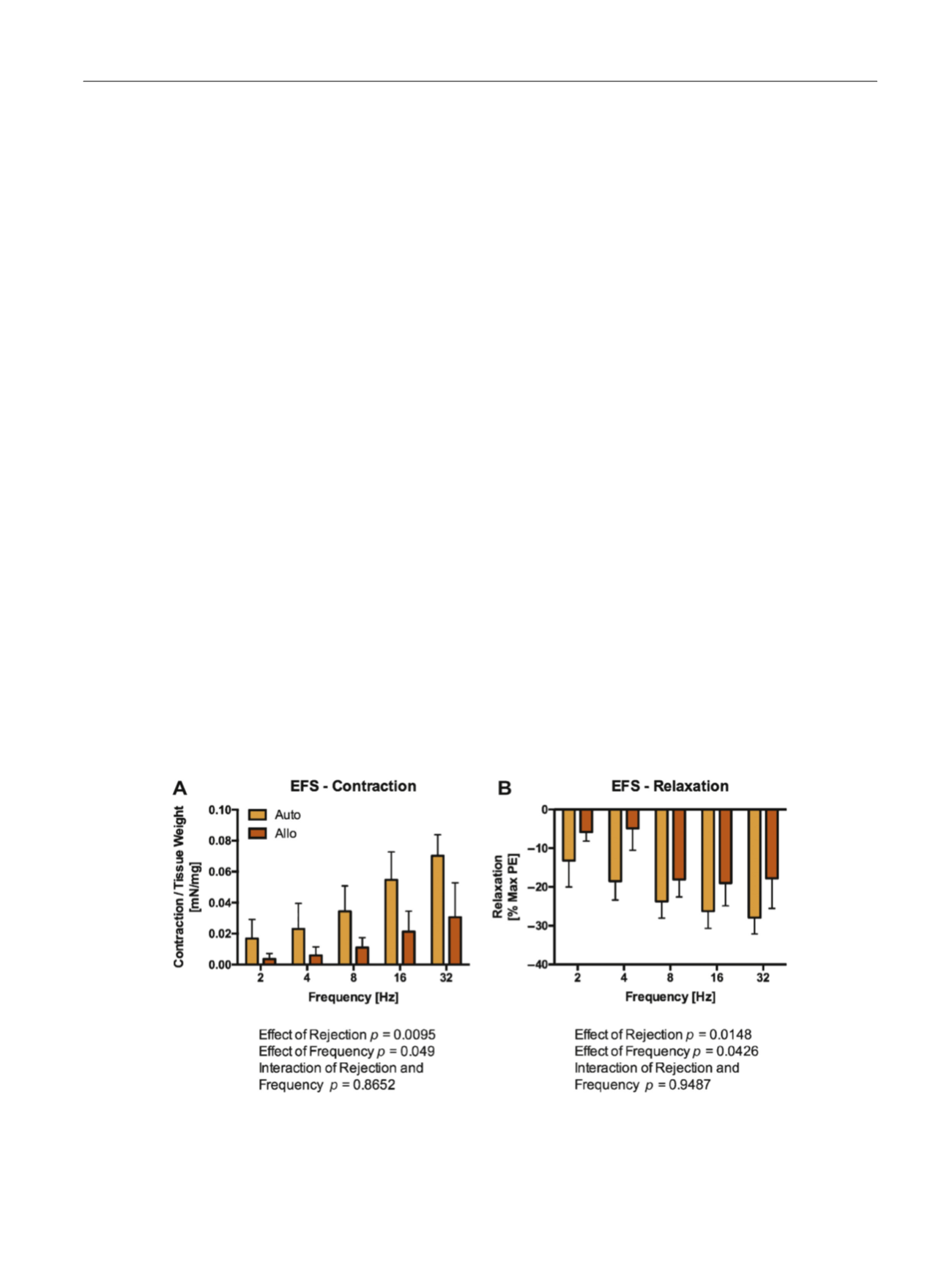
Having shown that an ex vivo rejection reaction occurs,
we next investigated the effects of rejection on cavernous
tissue physiological function as measured by tissue myo-
graphy. Following 48 h of culture in the MLR, EFS, which
relies on intact signaling between the cavernous nerves and
smooth muscle tissues, was assessed
( Fig. 2 ). Rejecting
tissues (cavernous tissues cultured with allogenic PBMCs)
demonstrated impaired EFS-mediated contraction compared
[(Fig._2)TD$FIG]
Fig. 2 – Rejection impairs nerve-mediated cavernous tissue contraction and relaxation induced by electrical field stimulation. Tissue myography was
performed on penile tissues following incubation for 48 h in either autologous or allogenic mixed lymphocyte reaction (Allo). A) Allo samples
demonstrated impaired nerve-mediated smooth muscle contraction. B) Allo tissues demonstrated impaired nerve-mediated smooth muscle relaxation
induced by EFS following maximal precontraction with phenylephrine. These data suggest that rejection impairs both the cavernous nerves and the
smooth muscle tissue of the penis, resulting in impaired tissue function, including erectile dysfunction.
n
= 4 per group.
Allo = allogenic; Auto = autologous; EFS = electrical field stimulation; PE = phenylephrine.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 8 4 – 5 9 3
587
















