
manufacturer’s instructions, to identify cells undergoing
apoptosis
[14]. Ex vivo MLR was prepared as described.
Additional cavernous tissues were collected following ex
vivo MLR and fixed with 10% neural buffered formalin
before being paraffin embedded and then were sectioned at
5
m
m and histochemically stained with hematoxylin and
eosin, Masson’s trichrome, Giemsa, and terminal deoxynu-
cleotidyl transferase dUTP nick-end labeling (TUNEL).
Histochemically stained tissues were imaged using a Zeiss
AxioObserver microscope (Carl Zeiss Microscopy).
2.4.
Real-time polymerase chain reaction human transplant
profiler array
Following ex vivo MLR, PBMCs were isolated from the media
supernatant by centrifugation and snap frozen. Cells were
mechanically disrupted, and total RNA was isolated and
purified using column separation (Qiagen,
Hilden,
Germany). Complementary DNAwas synthesized via reverse
transcription using the RT
2
First Strand Kit (Qiagen).
A human transplant polymerase chain reaction (PCR)
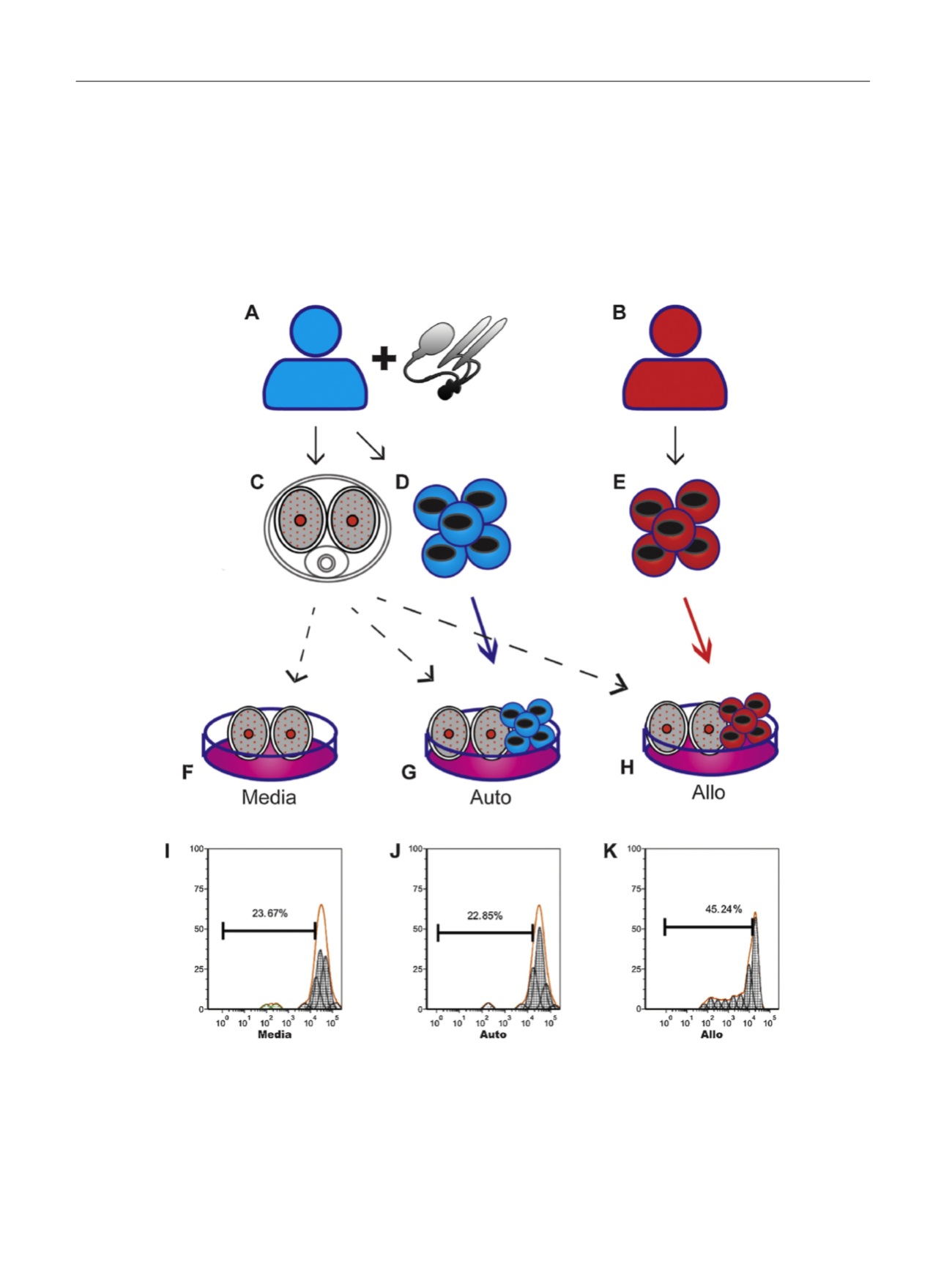
[(Fig._1)TD$FIG]
Fig. 1 – Schematic of human cavernous tissue mixed lymphocyte reaction cultured for 48 h in media. (a–e) During (a) penile prosthesis surgery, (c) ‘‘donor’’
cavernous tissue and (d) donor peripheral blood mononuclear cells (PBMCs) are taken from patients; (b) from a healthy volunteer, (e) ‘‘recipient’’ PBMCs
are taken. These materials are combined in various combinations to model human cavernous tissue transplant. (f) Cavernous tissue (pieces measuring
approximately 1
T
2 cm) is cultured in media as a nontransplant control reaction (Media). (g) Cavernous tissue and autologous PBMCs are cultured as an
autotransplant control reaction (Auto). (h) Cavernous tissue from the donor is cultured with allogenic PBMCs from the recipient to model tissue rejection
(Allo). (i–k) Representative proliferation plots of 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled PBMCs. Proliferation is assessed by
CFSE signal loss in daughter cells, which have decreased dye content due to mitosis, seen as the left-shifted peaks, with less CFSE intensity as the parent
peak. (i) PBMCs in media without penile tissue demonstrated the baseline proliferative rate of PBMCs in culture. (j) Donor PBMCs cultured with
autologous penile tissue demonstrated a proliferation rate similar to PBMCs cultured in media (i), suggestive of no rejection reaction occurring. (k)
Recipient PBMCs cultured with donor penile tissue demonstrated increased proliferation indicative of activation due to tissue rejection.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 8 4 – 5 9 3
586
















