
with the development of distant metastases, CSM, or ACM in
the overall cohort, or among patients with radiographic
lymphadenopathy (cN1; Supplementary Tables 5 and 6).
As a secondary analysis, we examined the association of
the number of LNs removed with oncologic outcomes
( Table 4). There was no significant association between the
number of LNs removed and the development of distant
metastases, CSM, or ACM on multivariable analyses, nor
when patients were stratified by cN/pN status
( Table 4 ,Supplementary Figures 2A–C).
4.
Discussion
In this study, LND was not associated with improved
oncologic outcomes in the overall cohort. More importantly,
we were unable to identify a high-risk population that
derived an oncologic benefit from LND, either among
patients with radiographic lymphadenopathy or when
systematically examining increasing thresholds of risk for
pN1 disease. Furthermore, among patients undergoing LND,
the extent of dissection was not associated with improved
survival. Taken together, these findings suggest that LND at
the time of RN does not confer a therapeutic benefit for M0
patients. Despite this, LND may still play a role in disease
staging, and, notably, has not been associated with
increased morbidity
[4,9].
The rationale for a potential oncologic benefit to LND in
RCC is based on the premise that complete resection may be
curative in cases where disease is limited to the LNs (ie,
lymphogeneous spread), and that cytoreduction in the
presence of occult systemic disease may improve response
to systemic therapy and overall oncologic outcomes
[18]. Indeed, durable long-term survival has been reported
in a subset of patients with isolated LN metastases
[5,19]. Still, the only randomized trial to examine LND,
European Organization for Research and Treatment of
Cancer
[42_TD$DIFF]
(EORTC) 30881, reported no difference in progres-
sion-free or overall survival, although it has been criticized
for its low-risk patient cohort and low incidence of nodal
metastases (4%)
[4] .In principle, LND is not expected to confer a therapeutic
benefit in the absence of nodal disease, and retrospective
studies have supported this in patients with clinically
negative nodes, consistent with
[43_TD$DIFF]
EORTC data
[10,20]. In
higher-risk patients, observational data dating back to the
1990s have suggested a survival advantage to LND
[9,11,12,21] .For example, Pantuck et al
[10]reported that,
among patients with clinically enlarged LNs, LND was
associated with improved survival. In the metastatic
setting, there is also indirect evidence supporting an
oncologic benefit to LND. Vasselli et al
[7]reported that,
among patients undergoing cytoreductive nephrectomy,
survival of patients with completely resected lymphade-
nopathy was similar to that of patients without lymphade-
nopathy. More recently, however, Feuerstein and colleagues
[22,23]did not identify a benefit to LND in either
nonmetastatic or cytoreductive settings.
Several studies have also examined whether the extent
of LND impacts oncologic outcomes. One population-based
study reported improved CSS with increased LN yield
among node-positive patients
[8], although methodological
concerns have been raised regarding the analysis
[24]. An-
other study reported improved CSS with a greater extent of
LND among patients with pT2 tumors, pT3c-pT4 tumors, or
tumors with sarcomatoid features
[13] .Our results are consistent with randomized trial data,
even among high-risk patient groups. There are several
potential explanations to reconcile the disparate findings
with prior retrospective studies. Most importantly, prior
studies employed less complete statistical adjustment and
may reflect selection bias and residual confounding. Indeed,
RCC has historically been associated with predominantly
hematogeneous, rather than lymphogeneous, spread
[9,25]. Rates of LN involvement among patients with
clinically nonmetastatic RCC (N1M0) are low—approxi-
mately 2–5%
[5,19,26] .Conversely, concurrent distant
metastases are present in approximately 58–67% of patients
with N1 disease
[10,27]. Moreover, there is biologic basis for
early hematogeneous spread in the setting of lymphatic
involvement, including mapping studies reporting direct
lymphovenous communications to the renal vein and
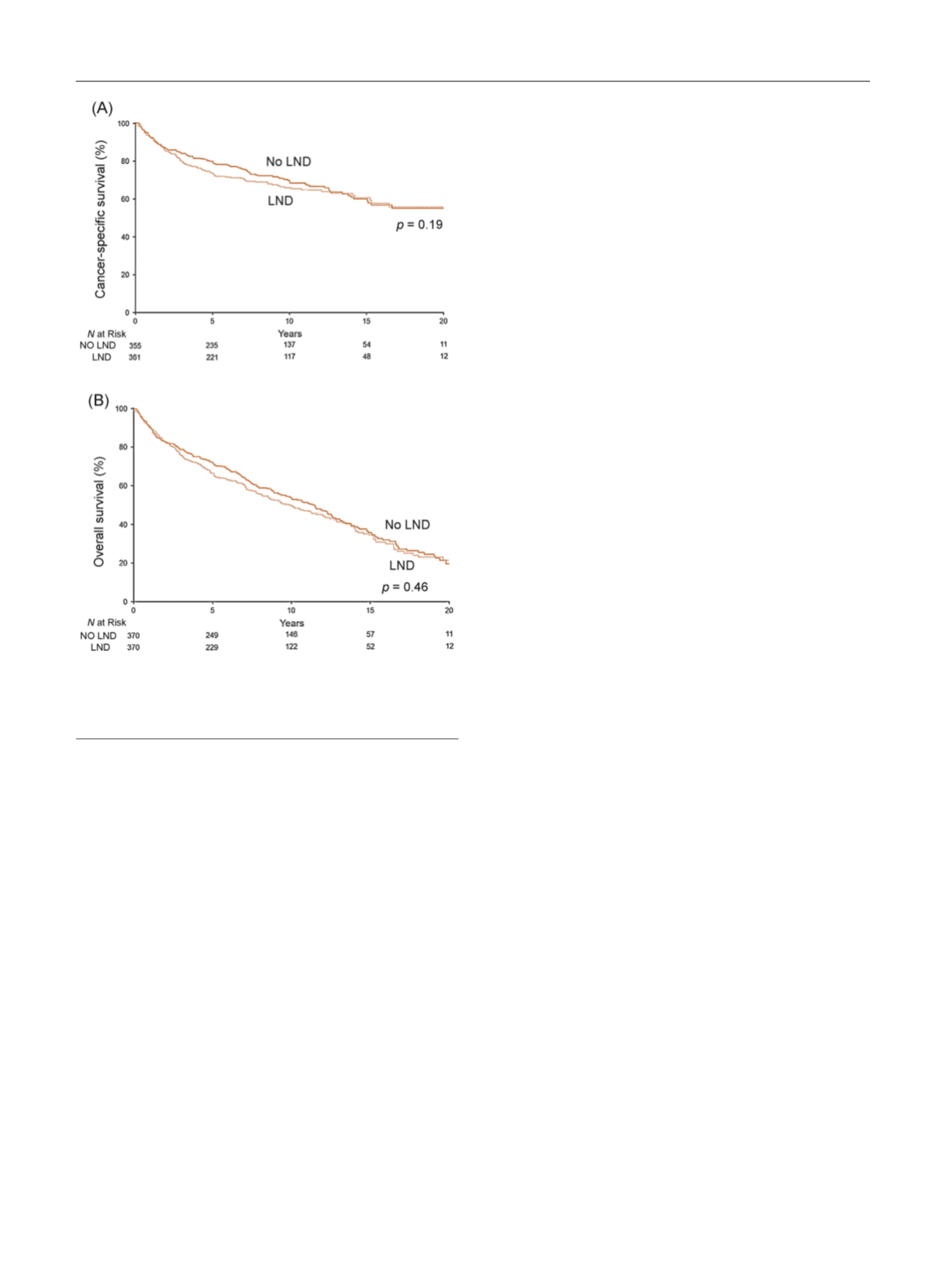
[(Fig._1)TD$FIG]
Fig. 1 – Association of lymph node dissection (LND) with (A) cancer-
specific survival among the subset of 370 propensity-score matched
pairs and with (B) overall survival among the subset of 370 propensity-
score matched pairs.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 6 0 – 5 6 7
564
















