
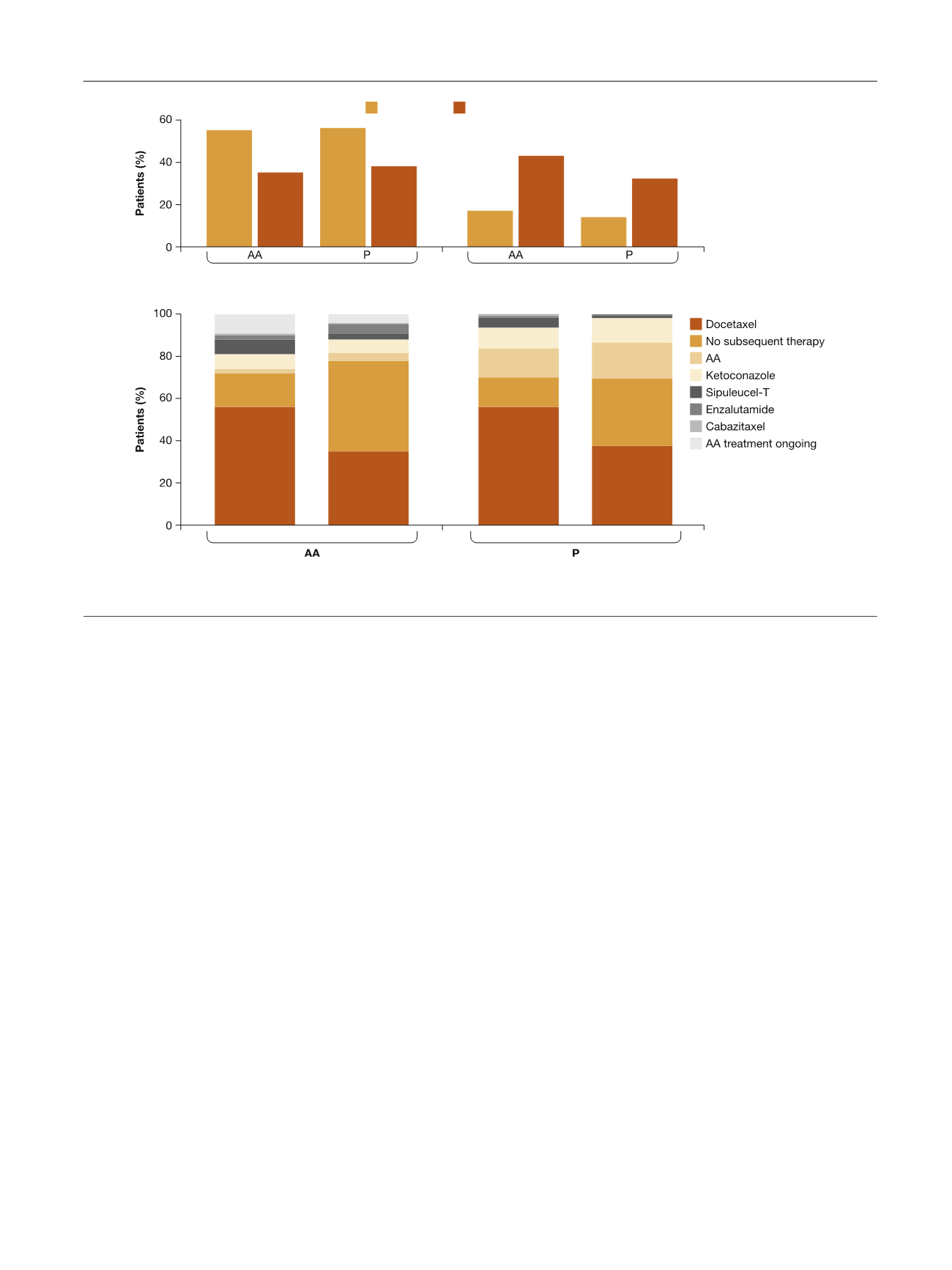
received AA before docetaxel (38% vs 63%;
p
= 0.02);
however, PSA responses to docetaxel were observed in
30% (7/23) of men previously treated with AA
[24]. In
addition, other reports have suggested no or minimal cross-
resistance between AA and docetaxel
[25]and between
ketoconazole and docetaxel
[26]. Additional results sup-
porting a clinical benefit for taxane-based chemotherapy
following AA were reported by Al Nakouzi et al.
[27]. In this
retrospective study of 79 patients with progressive mCRPC
after docetaxel and AA, PSA declines 50% were achieved in
35% (28/79) of patients who received subsequent therapy
with cabazitaxel
[27] .The potential role of AR splice variants as a resistance
mechanism is further evidence that all subsequent therapy
for mCRPC may not be effective
[28] .In a prospective study
of 62 men with mCRPC, detection of
AR-V7
mRNA in
circulating tumor cells was associated with resistance to AA
and enzalutamide
[29] .Results from two retrospective
studies suggest that the effects of AA following enzaluta-
mide treatment for mCRPC are associated with limited
response rates for chemotherapy-pretreated and chemo-
therapy-naı¨ve men
[30,31]. Similar observations were
reported for enzalutamide following AA treatment
[32]. However, a recent report suggests that AR-V7 is not
associated with primary resistance to taxane chemotherapy
[33]. Thus, it is plausible that some patients in the current
analysis progressed on AA treatment because of AR-V7, but
retained sensitivity to docetaxel. Overall, our results
suggest that a proportion of AA-unresponsive patients
may still derive a benefit from subsequent therapy with
docetaxel.
The treatment of mCRPC is evolving rapidly and there
may be geographic differences in terms of regional practice
patterns and available agents. While COU-AA-302 was an
international study, the availability of other drugs approved
for mCRPC (including enzalutamide, radium-223, and
cabazitaxel) varied by country, and this may have
influenced post-AA treatment patterns. In addition, subse-
quent to the conclusion of COU-AA-302, information from
two data sets emerged to support the use of upfront
docetaxel in the metastatic hormone-sensitive setting. The
impact of docetaxel in this earlier application on post-AA
treatment patterns and treatment efficacy will need to be
evaluated in future studies.
A substantial proportion (43%) of patients aged 75 yr
who progressed with AA received no subsequent therapy
with mCRPC drugs, suggesting that treatment nihilism may
exist, in part potentially because of the toxicity profile of
docetaxel in this population, although patient acceptance
and other disease characteristics may also be factors
[34,35]. Although the proportion of older patients receiving
no subsequent therapy is high, this finding is consistent
with other observations of treatment patterns among
elderly men with mCRPC
[36,37]. Interestingly, a high
proportion of patients in the AA treatment arm received
subsequent therapy, suggesting that patients remained fit
enough for subsequent therapy after progression on AA.
Overall, these observations suggest that the favorable
[(Fig._3)TD$FIG]
<
75 yr
≥
75 yr
<
75 yr
≥
75 yr
<
75 yr
≥
75 yr
Docetaxel as first subsequent therapy
No subsequent therapy
A
B
Fig. 3 – First subsequent therapy by age subgroup. (A) Docetaxel as first subsequent therapy and no subsequent therapy. (B) All first subsequent
therapy. AA = abiraterone acetate plus prednisone; P = placebo plus prednisone.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 6 5 6 – 6 6 4
661
















