
suggest that docetaxel may still impact clinical benefit in
the post-AA setting. The median TTPP was 7.6 months
which would be, similar to that from contemporaneous
reports of AA-naı¨ve patients treated with docetaxel in large
phase 3 trials
[19–21]. However, this observation needs to
be interpreted with caution owing to the high censoring
rate (71%), which is likely to have led to overestimation of
this value. Moreover, the median duration of docetaxel
therapy was based on patients with known docetaxel start
and end dates, whereas the median number of docetaxel
courses administered may not have been captured. With
this consideration, the median treatment duration in the
100-patient cohort described here was 4.2 mo, compared to
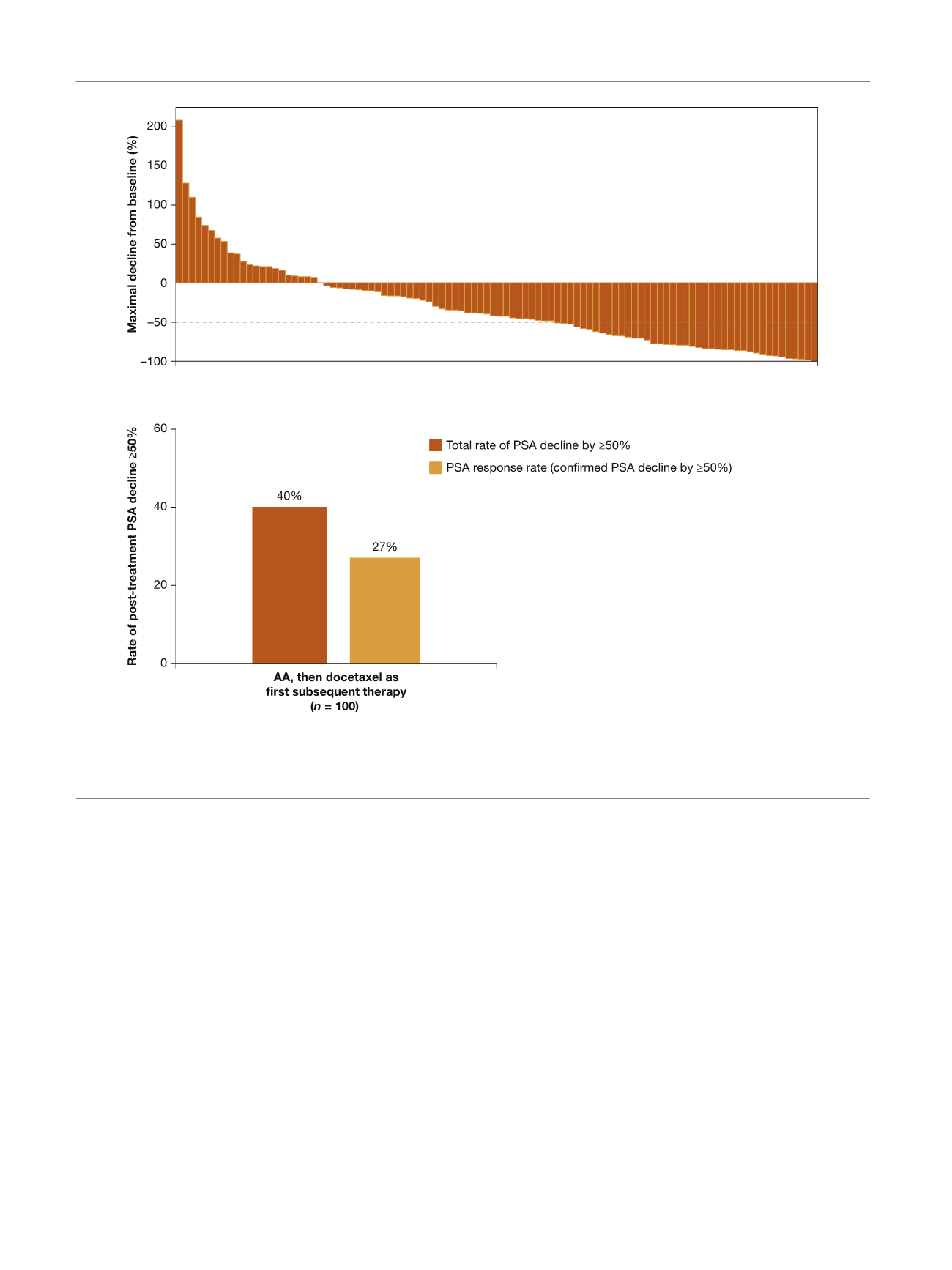
7.7 mo in the TAX-327 trial. The confirmed PSA response
rate among patients from the AA arm who received
docetaxel as FST was 27%, which is lower than the 45%
rate reported for docetaxel therapy for mCRPC in the phase
3 TAX-327 study
[4]. However, the rate of confirmed and
unconfirmed post-treatment PSA decline 50% was 40%,
which is closer to the TAX-327 findings. In addition, patients
in TAX-327 may have been vigorously selected and
prescreened in terms of performance status and prognosis
as part of the eligibility criteria for the trial, which
specifically investigated docetaxel use.
There is conflicting evidence that mCRPC patients who
experience disease progression after androgen signaling–
directed therapy may be less responsive to taxane-based
chemotherapy. Such cross-resistance could possibly be
mediated in part by taxane-induced disruption of androgen
receptor (AR) trafficking along microtubules
[22]. Results
from two retrospective studies suggest partial cross-
resistance between AA and docetaxel. In a study by
Mezynski et al.
[23], subsequent therapy with docetaxel
resulted in PSA declines 50% in 26% of cases and a median
TTPP of 4.6 mo (95% CI, 4.2–5.9) among mCRPC patients
previously treated with AA (
n
= 35). In a second study
[24],
mCRPC patients who received AA before docetaxel (
n
= 24)
had median PFS of 4.1 mo compared to 6.7 mo in the
docetaxel-only group (
p
= 0.002). In the same study, PSA
declines 50% were less frequent among patients who
[(Fig._2)TD$FIG]
A
B
Fig. 2 – Unconfirmed PSA declines among patients treated with abiraterone acetate who received docetaxel as first subsequent therapy. (A) Maximum
PSA decline from baseline. (B) Total and confirmed post-treatment PSA decline. Waterfall plot with maximum PSA change and PSA response rate for
patients with available baseline PSA within 30 d of subsequent docetaxel therapy and at least one post-baseline PSA value. PSA = prostate-specific
antigen; AA = abiraterone acetate plus prednisone.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 6 5 6 – 6 6 4
660
















