
identified five longer duration ( 1 yr) observational
studies reporting AEs.
3.2.
Silodosin versus tamsulosin
Ten trials randomized men with LUTS attributed to BPH
(
n
= 1799) to silodosin 8 mg daily versus tamsulosin
0.2–0.4 mg daily
[13–22].
Table 1provides baseline char-
acteristics. Overall, the RoB was low in two trials
[13,18],
moderate in six trials
[14–16,20–22], and high in two trials
[17,19] .Three trials conducted responder analyses (defined as
>
25% reduction in I-PSS score;
Table 2)
[16,18,20] .Response
to treatment was not significantly different between
silodosin and tamsulosin (risk ratio: 1.07, 95% confidence
interval [CI]: 0.91–1.26; moderate SOE). Silodosin and
tamsulosin (all dose levels) were not significantly different
in improving mean I-PSS scores (weighted mean difference
[WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE).
Results were similar in analyses restricted to trials using
tamsulosin 0.4 mg.
Overall withdrawals (for any reason) and rates of
participants with 1 AEs were not significantly different
between treatments (low and moderate SOE, respectively).
Withdrawals due to AEs were more frequent for silodosin
versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71;
moderate SOE). The most common AE was abnormal
ejaculation, reported in 16% of patients on silodosin versus
2% of patients on tamsulosin.
3.3.
Darifenacin plus ABs versus ABs alone
Two 146 12-wk trials (
n
= 161) compared darifenacin/AB
combination 147 therapy with AB monotherapy in men
with LUTS and overactive bladder (OAB) symptoms
attributed to BPH
[23,24]. Participants with a baseline
postvoid residual of
>
150 ml were excluded. RoB was low in
one trial
[24]and moderate in the other
[23] .SOE was
judged insufficient for all outcomes.
3.4.
Fesoterodine plus ABs versus ABs alone
Two trials (
n
= 990) compared fesoterodine/AB combination
therapy with AB monotherapy
( Table 1) in men with LUTS
and OAB symptoms
[25,26]. Overall RoB was moderate for
one trial
[25]and high for the other
[26]. Improvement in
mean I-PSS scores was similar with fesoterodine/AB
combination and AB monotherapy (low SOE;
Table 3).
The mean difference in the large moderate RoB trial was 0.0
(95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for
the small high RoB trial. Overall withdrawals, withdrawals
due to AEs, and the number of participants with 1 AE were
more frequent in the fesoterodine arm; SOE was judged as
low for all three outcomes.
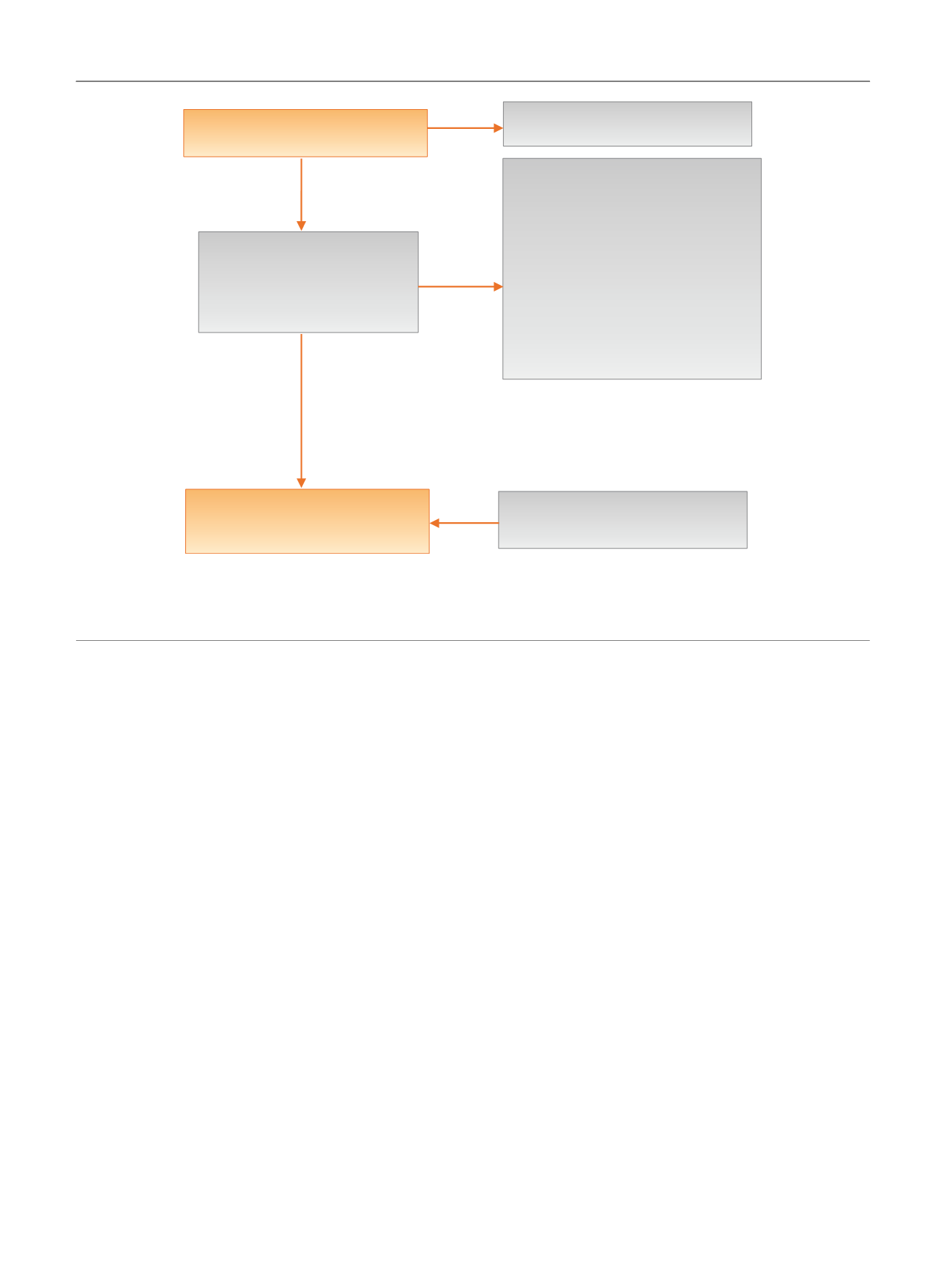
[(Fig._1)TD$FIG]
Title and abstract review excluded:
1162
Bibliographic database searches:
1310 references
Excluded:
105 references
Dup/secondary reports = 28
Not RCT = 26
Not head-to-head trial = 16
Not BPH = 7
Inadequate duration = 5
Not available in English = 2
No outcomes of interest = 9
Not valid comparison = 12
148 retrieved for full text
review
Eligible: 43 references
43 unique RCTs
5 unique observational studies
Harms search
5 unique observational studies
Fig. 1 – Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3
agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions
are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 7 0 – 5 8 1
572
















