
scores. Moreover, Shore et al
[10]reported that compared
with bicalutamide, a significantly higher proportion of
enzalutamide-treated patients demonstrated improvement
at any time during the study in seven FACT-P scales. The
HRQoL benefits of enzalutamide in our analyses occurred
despite a higher unadjusted proportion of patients
experiencing fatigue on enzalutamide (28% vs 20% with
bicalutamide)
[10].
In longitudinal analyses, there is no universally accepted
method for handling missing data as each method requires
assumptions about the nature of missing values. Formally
distinguishing between MAR and missing not at random is
not trivial and relies on untestable assumptions. A primary
analysis alone is insufficient when there are substantial,
nonrandom missing data. In TERRAIN, 40–55% of patients
discontinued treatment because of disease progression. For
the primary analysis, we used MMRM, which assumes that
missing data follow the pattern of patients who remained on
study. For the sensitivity analysis, we used PMM with
sequential modelling with multiple imputation when
imputation varies by reason for treatment discontinuation,
which considers that data may not be MAR. For FACT-P total
score, FAPSI-8, and EWB, results were similar using both
methods
( Fig. 1), suggesting that our findings were not
dependent on the nature of missing data for these outcomes.
Our results are consistent with the placebo-controlled
PREVAIL trial
[3,7]and confirm the HRQoL benefits of
enzalutamide in chemotherapy-naı¨ve patients with mCRPC.
HRQoL benefits in chemotherapy-naı¨ve mCRPC populations
have also been reported with abiraterone
[15]. Furthermore,
enzalutamide and abiraterone have shown HRQoL benefits
in mCRPC after chemotherapy
[6,8]. Moreover, TERRAIN is
the first study to directly compare two active treatments in
mCRPC, therefore reporting, for the first time, the effects of
two active mCRPC treatments on HRQoL.
HRQoL analyses can reinforce objective measures of
survival and radiographic progression
[3] ;improved HRQoL
has been linked to better clinical outcomes in mCRPC
[15]. In mCRPC, delaying HRQoL decline is an important
therapeutic objective in line with Prostate Cancer Clinical
Trials Working Group recommendations
[17].
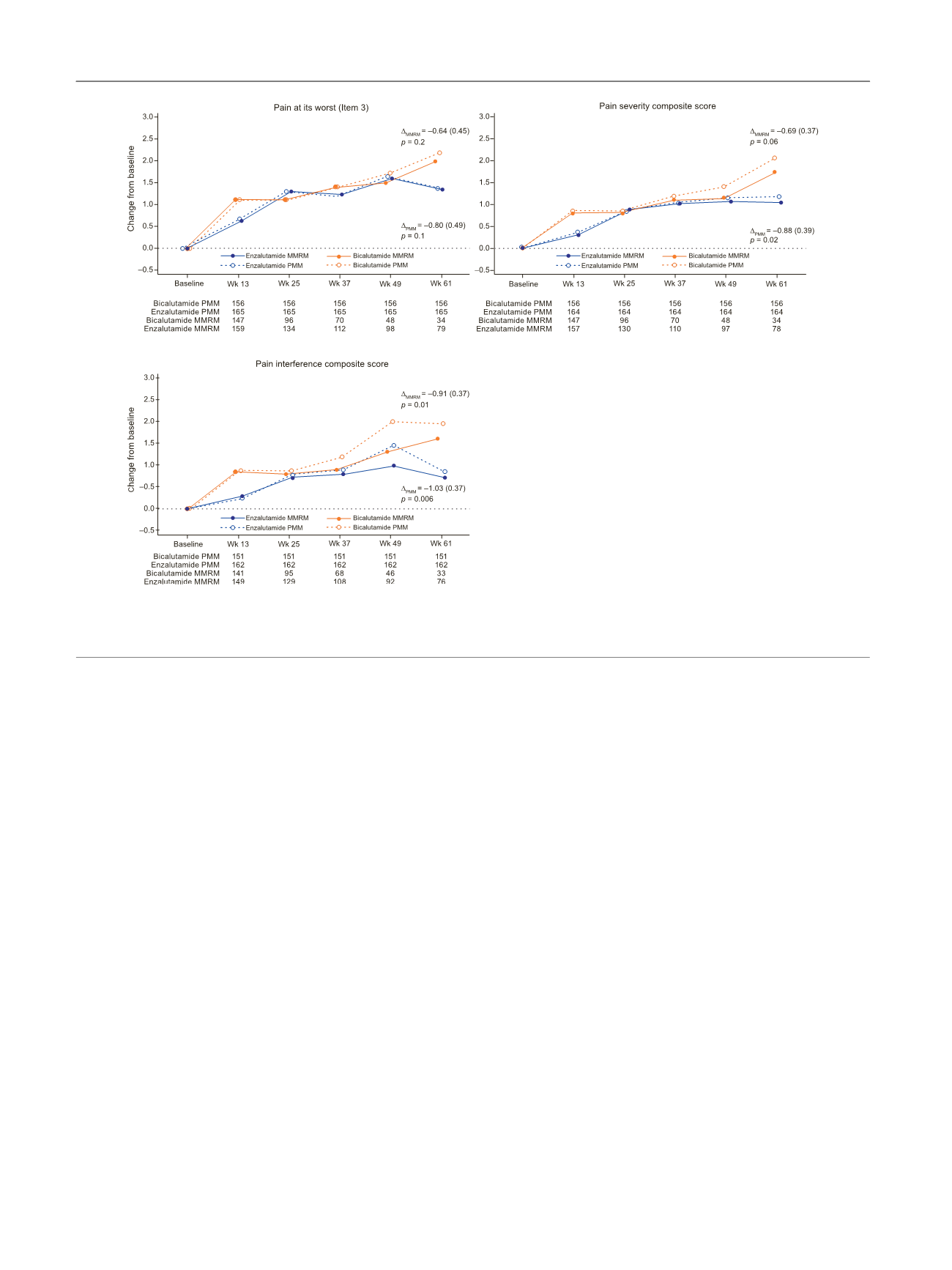
Pain is also a significant predictor of survival in mCRPC
[18] .While pain progression increasedwith both treatments,
patients on enzalutamide experienced a smaller increase
versus bicalutamide at 61wk, with a significant difference for
pain interference. There was no between-treatment differ-
ence in time to pain progression. In the PREVAIL trial in
chemotherapy-naı¨ve patients withmCRPC
[7], enzalutamide
delayed progression in BPI-SF pain at its worst and in average
pain severity and interference versus placebo.
Study limitations include the exploratory nature of the
HRQoL analyses, lack of multiple comparisons corrections,
and unknown effects of anxiety/depression on HRQoL.
Depression and anxiety in cancer
[19]can be associated
[(Fig._3)TD$FIG]
Fig. 3 – Adjusted mean change (standard error) from baseline in Brief Pain Inventory, Short-form scores (mixed model repeated measures [MMRM] and
pattern mixture model [PMM] analyses for pain at its worst (Item 3), pain severity composite score, and pain interference composite score.
D
is the
adjusted mean (standard error) change from baseline with enzalutamide versus bicalutamide at wk 61 (MMRM or PMM).
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 5 3 4 – 5 4 2
540
















