
the PRECISE case report template form to report MRI at baseline or
follow-up in these men.
The checklist provides a guide for authors in preparing a manuscript
for publication and for reviewers and editors when assessing manu-
scripts. The case report template form is suitable for clinical use allowing
communication of imaging findings and their likely relevance to
referring clinicians, and it will also allow data collection to inform the
reporting of cohorts of men.
2.2.
Setting and participants
The panel included 10 experts in urology, 8 in radiology, and 1 in
radiation oncology (Supplementary
Table 1summarises panellist
experience). Faculty attending the 2nd European School of Oncology
Active Surveillance February 2016 workshop in Milan, Italy, were
initially approached to join the panel. Additional members not attending
the workshop were invited to ensure a balance of expertise. Two panel
members were unable to travel to the meeting and participated by
online conference (B.T. and P.P.) with audio participation and desktop
viewing so they could see all of the presentations.
3.
Results
To avoid ambiguous statements and to identify consensus if
it existed, 38 statements were deleted, 56 statements
modified, and 11 statements added during the panel
meeting, giving a final set of 367 statements that were
scored.
During the first round, 201 of 394 statements were
scored with consensus and agreement.
Table 1shows the
scoring during the meeting.
3.1.
The PRECISE case report form for reporting a magnetic
resonance study in an individual man on active surveillance
The PRECISE case report form
( Fig. 2) includes each item
that should be reported for an individual man having anMRI
at baseline or follow-up during active surveillance.
3.2.
The PRECISE checklist for reporting cohorts of men having
magnetic resonance imaging in active surveillance
The PRECISE checklist
( Table 2 )shows the panel recom-
mendations for reporting a cohort of men who have a
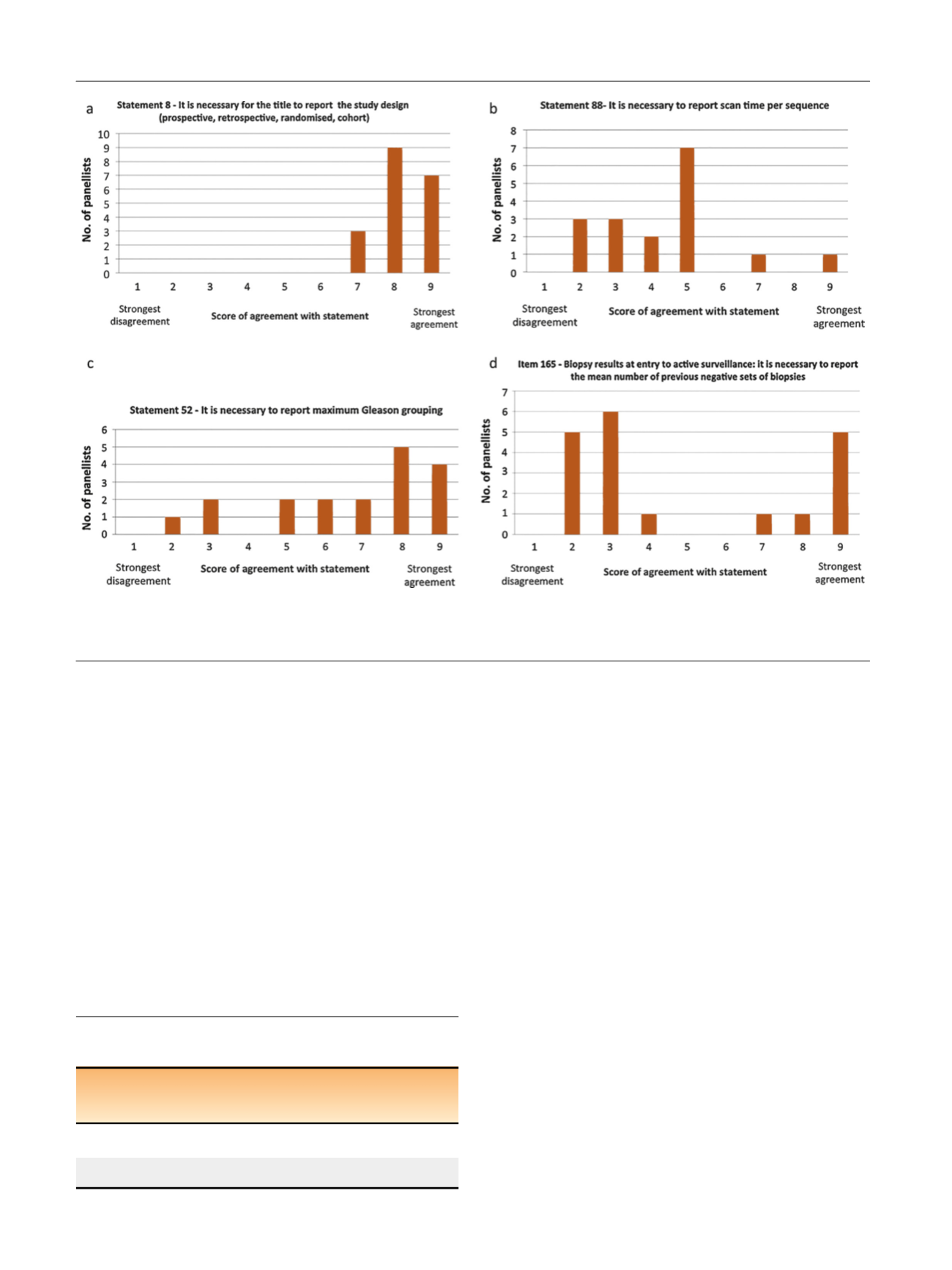
[(Fig._1)TD$FIG]
Fig. 1 – Graphic representation of the group response for four statements showing (a) agreement and consensus (group median score: 8), (b)
uncertainty and consensus (group median score: 5), (c) agreement and no consensus (group median score: 7.5), and (d) disagreement and no
consensus (group median score: 3).
Table 1 – Summary of the group responses before and during the
meeting
Agreement
and consensus,
n
(%)
Disagreement
and consensus,
n
(%)
Uncertainty
or no consensus,
n
(%)
Before meeting
(
n
= 394)
201 (51)
12 (3)
181 (46)
During meeting
(
n
= 367)
144 (39)
34 (9)
189 (52)
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 6 4 8 – 6 5 5
650
















