
prostate MRI during active surveillance. All statements in
the checklist were scored with consensus and agreement.
Items were not included in the checklist if they were
scored with disagreement or lack of consensus at the
meeting. Items were grouped together, and all definitive-
ly agreed statements were included. Supplementary
Table 2describes the full list of items and their scores.
The intention was to develop a comprehensive but not
restrictive set of statements, balancing the need for
clarity and brevity and recognising the variations in
current reporting practice, both in histologic and radio-
logic data.
3.3.
Reporting the conduct of magnetic resonance imaging
The PRECISE guidelines are not intended to replace or
compete with the comprehensive guidelines on the conduct
of prostate MRI developed by the Prostate Imaging
Reporting and Data System (PI-RADS) group
[4]. The panel
agreed that publications should state whether study MRI
scans were conducted in accordance with contemporary
guidelines and should cite the guidelines used. We
recognise that the conduct of MRI may change over the
reporting period of a study because of the longitudinal
nature of active surveillance cohorts.
3.4.
Reporting of magnetic resonance imaging
The number of radiologists reporting scans in the study
cohort should be stated. If an individual scan was reported
by more than one radiologist, the use of separate or
consensus reporting should be clarified. When scans were
reported separately, the method used to combine results
should be used (eg, mean of absolute size values at each
time point, mean change in size between scans per
reporter). The format of the radiology report should be
stated (eg, prose, template, and/or diagrammatic reporting,
with and without embedded or annotated MRI images). The
PRECISE case report form was designed to facilitate the
routine collection of clinical and imaging data in a manner
that will allow cohort comparison of men on active
surveillance in a standardised manner. It should be stated
whether the MRI readings were done retrospectively, with
one reading of a set of MRIs from previous time points, or
whether scans were reported contemporaneously, with or
without reference to previous images or reports.
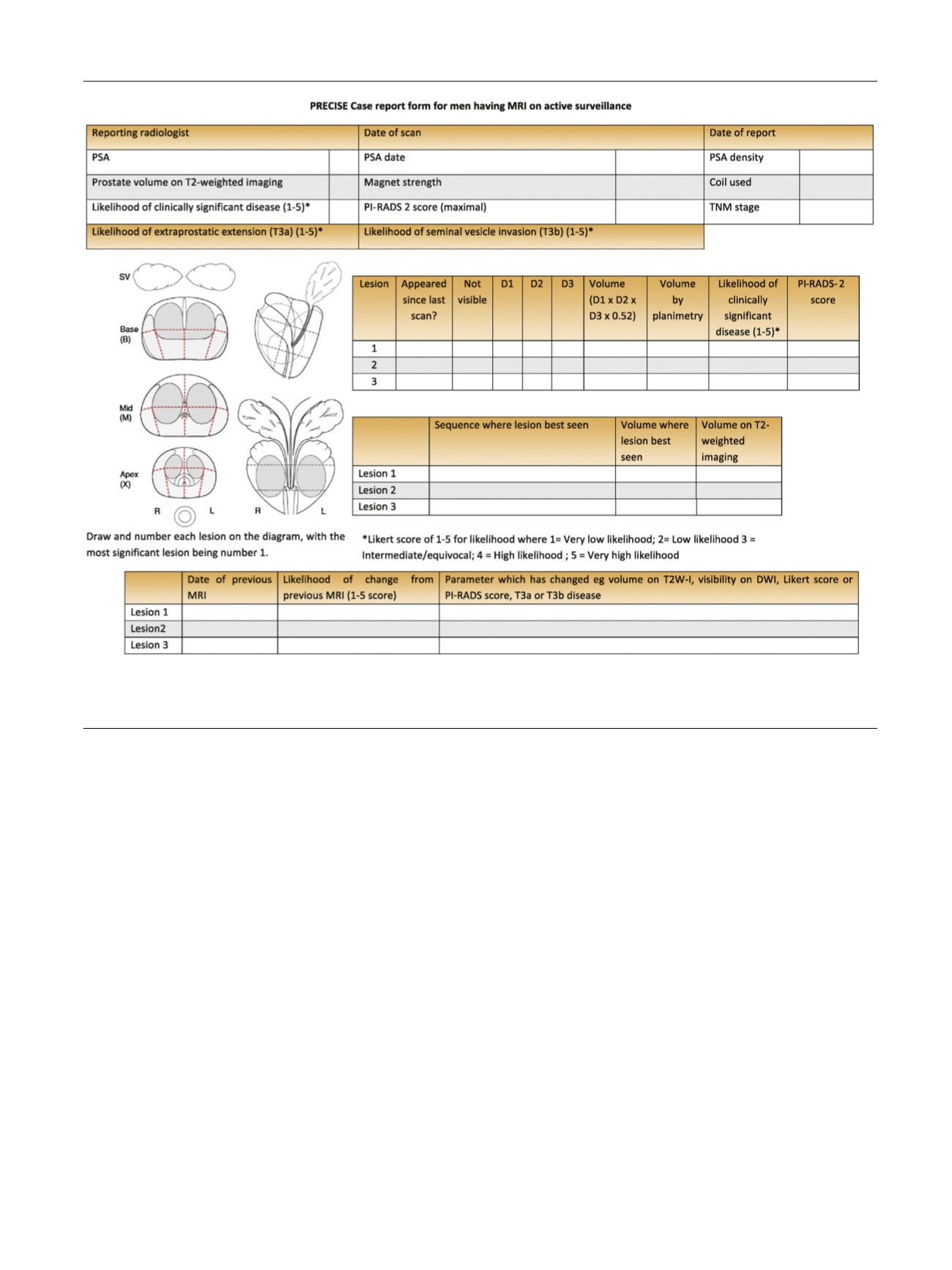
[(Fig._2)TD$FIG]
Fig. 2 – Case report form for reporting of magnetic resonance imaging at baseline and during follow-up in men on active surveillance.
MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging Reporting and Data System; PRECISE = Prostate Cancer Radiological Estimation of
Change in Sequential Evaluation; PSA = prostate-specific antigen; T2W-I = T2-weighted image.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 6 4 8 – 6 5 5
651
















